The paper in Nature Ecology & Evolution is here: http://go.nature.com/2uYUl5x
All that I am, or ever hope to be, I owe to my angel mother. These words by Abraham Lincoln capture the essence of our paper. Life would be impossible without mitochondria, the tiny ‘power plants’ of our cells, and we owe them all to our mothers; mitochondria and their genome are transmitted only from mothers to offspring (with rare exceptions like mussels). Yet, the word ‘angel’ is perhaps to reconsider in Lincoln’s quote because, as we write in our paper opening, “mitochondria could be poisoned gifts that mothers transmit to their sons”…
Imagine a mitochondrial genetic variant that decreases the fitness in males only, with no effect in females. For instance, fewer males might survive to reproduce among those who carry that variant than among those who do not. Thus, the variant is under negative selection through one sex only. But here’s the rub: natural selection will be inefficient at decreasing its frequency because this frequency will depend on the reproduction by those individuals who can effectively transmit the variant to their progeny, namely females. Hence the expression “mother’s curse” (MC) coined to designate the particular form of intragenomic sexual conflict involving mitochondria.
How bad can this be for males? Well, we have no clear answer yet, but traits showing a male-biased expression, such as sperm production, or those for which optima may differ between the sexes, such as lifespan, should be the most susceptible to the mother’s curse. Strong experimental evidence supporting this prediction comes from recent Drosophila studies (E.g. Camus et al. (2012) Curr Biol 22, 1717-1721; Innocenti et al. (2011) Science 332, 845-848; Patel et al. (2016) eLife 5, e16923), although only a handful of mutations potentially exposed to MC have been detected so far.
Nuclear genes can give a hand to reduce the detrimental effects of mitochondrial variants, though. After all, their transmission by affected males is compromised. They must counterattack (as selfish genes) or, even better, cooperate with mitochondria to come up with nuclear-mitochodrial allele combinations that maximize the fitness of both genomes (since both contribute to the molecular machinery that made up the cellular respiratory chain, some cooperation is a necessity). Indeed, these mitonuclear interactions (and other compensatory mechanisms) must have been important over the course of evolution, otherwise mtDNA would accumulate deleterious mutations to the extent of causing species extinction, at least in theory.
We yet know little about how mitonuclear interactions shape phenotypes but an abundant medical literature underscores their role in the variable penetrance of genetic diseases. One of them, the Leber hereditary optical neuropathy (LHON), is a mitochondrial disease that predominantly causes vision loss, although various complications can arise in both men and women, such as encephalopathies and cardiopathies. Due to the extreme sex bias in its expression (about eight males affected for every one female among carriers of the causal mutation in our study population), LHON is viewed as the archetypal example of the mother’s curse.
However, the mother’s curse is essentially about fitness, not disease (you could die from the most dreadful disease expressed at a late age and still leave numerous descendants). Somehow, a disease must be associated with lower male fitness to merit the MC “trademark”. Thus, the most direct way to test the MC hypothesis is by measuring individual fitness (not symptoms) while following the frequency of the focal DNA variant over several generations. This is what we did.
The study was brewed from an assembly of researchers coming from varied disciplines: Damian Labuda and Claudia Moreau were working on human population genetics and epidemiology, Alain Gagnon on historical demography, Alan Cohen on epidemiology and biology of ageing, Bernard Brais on medical neurogenetics, while I was more on the evolutionary biology side, although there were areas of overlap in our respective fields of interest. Our meeting point was truly the exceptional historical records from the French-Canadian population: data on over 5 million individuals born or married in Québec since its colonization by French settlers, providing a nearly four century window to study the biological evolution of a large population since its foundation. So we designed our first joined project that end up having an evolutionary medicine flavour, which was quite exciting.
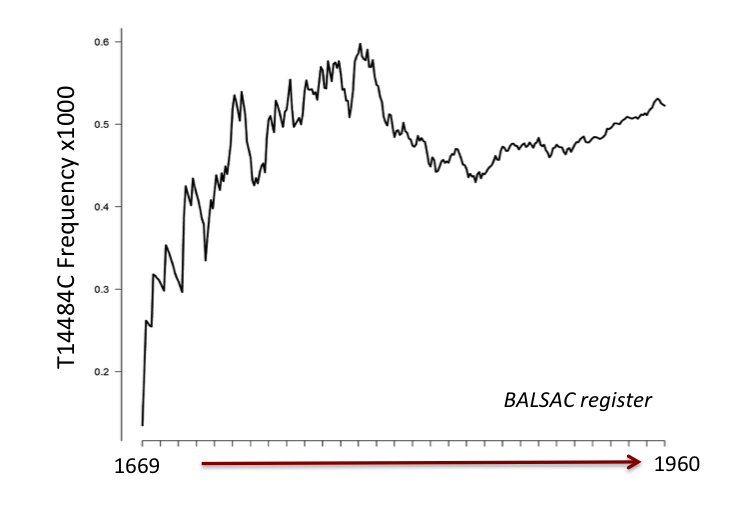
But credit must be given here to the authors of a pioneer study published in 2005 in the American Journal of Human Genetics by Laberge et al. and conducted in Bernard’s lab. They had identified the French woman who introduced LHON in Québec some 350 years ago! Thus, this woman boarded on a boat in 1669 to cross the Atlantic and settle in the New World with the hope to find a better life there. She was one of several hundred single women (between 700 and 800) sent as bride to support the peopling of the young New France colony. She brought along the LHON-causing mutation T14484C and, using data from modern subjects and climbing back their genealogy, Laberge and coauthors had pinpointed a singular, yet consequential gene flow event in the history of Québec.

Years after Damian suggested that we joined our expertise to tackle the LHON case with an evolutionary biology approach. We found strong support for the MC hypothesis based on multigenerational fitness data from this natural (human) population. Our three main contribution/findings are summarized here:
- Using genealogical imputation, we identified 2038 T14484C carriers in Québec history and followed the fate of the mutation over 290 years. We are not aware of another study reporting such a population-wide coverage of the history of a human genetic variant;
- We find that the fitness of male carriers was much lower than that of female carriers, and showed that the mutation would have been rapidly purged from the population without the mother’s curse. Possibly, there was even a reinforcement of the MC effect due to a slight fitness advantage that female carriers had over non-carriers (a boosted mother’s curse!);
- Remarkably, the fitness cost in males was mediated mostly through infant mortality, not by adult reproductive performance. Such a population-wide association between a LHON-causing mutation and infant mortality is unknown in the medical literature. So, while the mother’s curse is generally viewed as an accumulation of mutations decreasing fecundity or accelerating senescence in male adults, in this study we find that MC on a single mitochondrial variant affecting infants had a outstandingly high impact on the fitness of males.
Recently I was presenting these results at a conference in Montreal when someone raised his hand and asked: “Are you aware that you are presenting about the mother’s curse on mother’s day?” Admittedly, timing could not be worse!
Emmanuel Milot




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in